1. Introduction
Plants produce a wide variety of compounds to sustain and support growth, development, and reproduction, including secondary metabolites that are not essential for plant growth and, in contrast to primary metabolites, typically bear complex structures. The precise composition and chemical complexity of secondary metabolites became known only with significant improvements with regard to analytical techniques in the middle of the 20th century; particularly the development of chromatography.1 Extractable plant secondary compounds constitute up to 30 % of the dry weight of terrestrial plants, especially in forest ecosystems,2 with their main role as defense against pathogens and herbivores,3 but also as allelopathic agents,4 antioxidants protecting leaves from UV radiation, and excess of light5as well as regulators of nutrient and carbon cycling.6
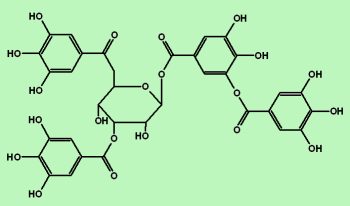
Among the vast amount of plant secondary compounds, tannins quantitatively dominate, representing the fourth most abundant group of compounds in vascular plant tissue after cellulose, hemicelluloses, and lignin.7 Plants can contain up to 20 % of their dry weight in tannins;7, 8 the amount, however, changes in response to environmental conditions.7 In turn, the effect of environmental stress, such as drought, on the production of tannins is complex and depends on further factors, for example the ontogenetic stage at which the drought stress occurs.9 Additionally, many studies found high tannin concentrations in plants occurring in habitats with low soil fertility and low pH.10 Moreover, it was shown that warming and altered precipitation can affect the chemistry of tannins by increasing their reactivity.11 Chemically, tannins are often divided into two main groups: hydrolysable tannins (HTs) and condensed tannins (CTs) (Figure 1). Hydrolysable tannins can be separated into gallotannins and ellagitannins built up from of gallic acid or hexahydroxydiphenic acid esters, respectively, linked to a sugar moiety (Figures 1 A and 1 B). Condensed tannins (proanthocyanidins) are polymers of three‐ring flavonols joined through C−C bonds12 (Figure 1 D). Monomers of CTs are divided into procyanidins and prodelfinidins (Figure 1 C). The newest findings point to a specific chloroplast‐derived organelle called tannosome as the location of tannin production at the cellular level,13, 14 from which tannins are transported to vacuoles. Overall, the chemical structure of tannins is plant species‐specific and shows a very high variability with probably no two species bearing the same tannin pattern;15 thus, studying tannin chemistry can be very challenging. However, the problem of methodological development is not be presented here.

Open in a separate window
Figure 1
Structure of tannins. A) simple gallotannin, B) simple ellagitannin, C) basic unit of condensed tannins, and D) condensed tannin trimer. Modified from Ref. 6.
In this Review, we focus on the recent novel insights into the chemistry of tannins, their interactions with other chemicals, and their influence on enzymatic activity. We challenge tannin chemistry paradigms with the newest findings to obtain a more holistic view on these plant secondary compounds. In Section 2, we evaluate the basic knowledge on tannin–protein interactions, adding the newest findings in the field. In Section 3, we challenge the traditional view on tannin chemistry, that is, that tannins are simply enzyme inhibitors. In Section 4, we expand the reactions of tannins to non‐protein N compounds, underlining the remarkable versatility of tannin chemistry.
2. Interaction of Tannins with Proteins
According to Bate‐Smith and Swain,16 tannins are “water‐soluble phenolic compounds, with a molecular mass between 0.5 and 3 kDa, able to precipitate proteins and alkaloids”. These tannin mass limitations (0.5–3 kDa) have been extended with time, as also lower and higher molecular mass polyphenolics are able to precipitate proteins;17 however, the ability to form complexes with proteins is still a unique characteristic of tannins,16 for example, used already in ancient times to produce leather from animal skin. The reaction between tannins and proteins involves two stages: first the binding and second the aggregation, resulting in the formation of the precipitate.18, 19, 20 Although earlier work on tannin–protein complexes pointed to non‐covalent bonding and insoluble precipitates, more recent studies add covalent bonding and soluble complexes as a possible result of interactions between tannins and proteins.16
The formation of tannin–protein complexes depends on numerous factors dominated by tannin and protein chemistry (e.g. proline content), concentration, protein isoelectric point, pH, and ionic strength of the solution and also presence of other compounds in the solution.21, 22, 23, 24, 25 The importance of the molecular complexity of tannins for a reaction with proteins was underlined by Haslam,18 who first used structurally well‐defined polyphenols and found that the most crucial features of tannins are phenolic sites crosslinked with proteins. Furthermore, proteins, which are especially prone to reactions with tannins, are proline‐rich proteins (PRPs) found in mammalian saliva.26 These interactions between PRPs and tannins protect dietary nitrogen from polyphenols, but also play a role in taste sensation known as astringency, a feeling of loss of lubrication and dryness.19, 27
According to a well‐known paradigm in tannin chemistry, precipitation of different proteins by tannins strictly depends on the protein isoelectric point (pI).21 At a pH close to the isoelectric point, proteins aggregate more eagerly because they carry no net electrical charge.21 However, according to the newest findings, tannins can also form complexes with proteins at a pH far from their isoelectric point.28, 29 Bovine serum albumin (BSA) typically used in tannin–protein interaction studies with pI 4.7 formed complexes with hydrolysable tannins at neutral pH28, owing to tannin oxidative activity.29 Although interactions between tannins and proteins have been intensively studied over the past 50 years, an in‐depth understanding of all mechanisms regulating tannin–protein interactions is still lacking.
3. Specific Interaction of Tannins with Enzymes
As the majority of enzymes belong to proteins, it is widely believed that tannins decrease enzymatic activity as a result of enzyme complexation.22, 30−32 Although studies over the past decades have established tannins as potential inhibitors of enzymatic activity,30, 32, 33 some studies found only a minor decrease in their activity.34, 35 Thus, our current understanding on their inhibiting role is still limited. Furthermore, the potential enhancement of enzyme activity by tannins has been overlooked for decades, with only very few exceptions. A study by Tagliazucchi et al.36 showed the ability of some phenolic compounds to enhance pepsin activity, which, however, was explained by phenolic‐induced changes in the substrate protein.37, 38, 39 Moreover, a highly heterogenic incubation study found that tannin‐rich leaves in nylon‐gauze bags in the rumen increased the activity of glutamate ammonia ligase, but no mechanical evidence was provided.40 Only recently, evidence has been found that enzymatic activity is increased after the reaction with tannins present in low concentrations (Figure 2 A).41 This study showed that low concentrations of tannins increased the coiled structures of the enzymes, thereby boosting their catalytic activity.41 High concentrations of tannins lead to opposite results by diminishing the catalytic activity (see Figure 2 C), although even enzyme–tannin complexes exert some residual activity (Figure 2 B).41 The response of enzymes to tannins varied depending on the enzyme.41 Overall, the interactions between tannins and enzymes follow the same rules as for tannins interacting with non‐enzymatic proteins (see Section 2). However, enzymes vary in their affinity to tannins; thus, the potential influence of unknown tannins on a given enzyme is unpredictable. Recent findings in this section suggest that tannins are more than just inhibitors, but rather modifiers of enzyme activity, which should raise interest in different fields controlling enzymatic activity, such as food chemistry, medicine and industry.

Open in a separate window
Figure 2
Influence of tannins on enzymatic activity of acid phosphatase: A) changes in enzymatic activity after addition of tannins in different concentrations, B) residual activity of enzymes after formation of complex with tannins, C) infrared spectra of enzyme secondary structure presented as a stacked plot of second derivative. Black lines represent enzyme without tannins and blue lines enzymes with low tannin concentrations. Red lines show enzymes with high tannin concentration. Spectra are smoothed by using eight points. Region of alfa‐helix marked in yellow. Modified from Ref. 42.
4. Tannin Interactions with Organic Non‐ Protein N Compounds
According to the definition by Bate‐Smith and Swain,16 tannins form precipitates with proteins, but also with alkaloids. However, tannins also create complexes with metals,16, 43 and other compounds, i.e., tannic acid (TA), a common hydrolysable tannin, forms complexes with choline, an amine precursor of acetylcholine;44 TA also adsorbs to chitosan.45, 46 It is widely assumed that tannins from the entire pool of organic N compounds precipitate only proteins/peptides.42 However, only recently, it was shown that tannins can react with a wide set of different organic N compounds,42 including arginine (from all amino acids), nitrogen bases, polyamines, chitin, and chitosan.42 Similarly to tannin–protein reactions, the concentration, chemical structure, and pH of the solution seem to play a decisive role.42 For example, the ability to form multiple hydrogen bonds47 facilitates the formation of complexes with tannins. For proteinaceous amino acids, polyamines, and nitrogen bases, a higher reactivity towards tannins was found with higher molecular masses and more amine groups:42 of all amino acids, arginine has the highest number of amine groups (4) and almost the highest molecular mass (174 Da); for polyamines, spermine has the highest molecular mass (202 Da) and amount of amine groups (4) (see Table 1). For nitrogen bases, the two having no amine groups exerted the weakest reactivity towards tannins.42 Thus, these findings on tannin–non‐protein interactions lead us to further emphasize the importance of tannin chemistry. Moreover, reactions with numerous N compounds call for a change in our way of thinking about tannins: they can react with non‐protein organic N compounds similarly to their reaction with proteins.
Table 1
Reactivity of different organic N compounds towards tannins. Modified from Ref. 42.
| Compound | Mw [Da] | N content [%] | Additional information (e.g. functional groups) | Reaction with tannins |
|---|---|---|---|---|
| Amino acids | ||||
| alanine | 89 | 15.7 | 1 amine, 1 methyl, 1carboxyl | − |
| arginine | 174 | 32.1 | 4 amine, 1 carboxyl | + |
| asparagine | 132 | 21.2 | 1 amide, 1 amine, 1 carboxyl | − |
| aspartic acid | 133 | 10.5 | 1 amine, 2 carboxyl | − |
| cysteine | 121 | 11.5 | 1 amine, 1 carboxyl, 1 thiol | − |
| glutamic acid | 147 | 9.5 | 1 amine, 2 carboxyl | − |
| glutamine | 146 | 19.1 | 1 amide, 1 amine, 1 carboxyl | − |
| glycine | 75 | 18.6 | 1 amine, 1 carboxyl | − |
| histidine | 155 | 27.0 | 1 imidazol, 1 amine, 1 carboxyl | − |
| isoleucine | 131 | 10.6 | 1 amine, 1 carboxyl, 1 methyl | − |
| leucine | 131 | 10.6 | 1 amine, 1 carboxyl, 1 methyl | − |
| lysine | 146 | 19.1 | 2 amine, 1 carboxyl | − |
| methionine | 149 | 9.3 | 1 amine, 1 carboxyl, 1 thiol | − |
| phenyl‐alanine | 165 | 8.4 | 1 amine, 1 carboxyl, 1 phenyl | − |
| proline | 115 | 12.1 | 1 carboxyl, 1 pyrrolidine | − |
| serine | 105 | 13.3 | 1 amine, 1 carboxyl, 1 hydroxyl | − |
| threonine | 119 | 11.7 | 1 amine, 1 carboxyl, 1 hydroxyl, 1methyl | − |
| tryptophan | 204 | 13.7 | 1 amine, 1 carboxyl, 1 indole | − |
| tyrosine | 181 | 7.7 | 1 amine, 1 carboxyl, 1 phenyl, 1 hydroxyl | − |
| valine | 117 | 11.9 | 1 amine, 1 carboxyl, 2 methyl | − |
| Polyamines | ||||
| putrescine | 88 | 31.8 | 2 amine | + |
| spermidine | 145 | 28.9 | 3 amine | + |
| spermine | 202 | 27.7 | 4 amine | + |
| N bases | ||||
| adenine | 135 | 51.8 | 1 amine, 4 N in heterocyclic ring | + |
| cytosine | 111 | 37.8 | 1 amine, 1 ketone, 2 N in heterocyclic ring | + |
| guanine | 151 | 46.3 | 1 amine, 1 ketone, 4 N in heterocyclic ring | + |
| uracil | 112 | 25.0 | 1 methyl, 2 ketone, 2 N in heterocyclic ring | + |
| thymine | 126 | 22.2 | 1 methyl, 2 ketone, 2 N in heterocyclic ring | + |
| Aminosugars | ||||
| chitin | (203)n | 6.89 | 2 amide, 4 hydroxylic, 2 methyl | + |
| chitosan | (161)n | 8.69 | 1 amine, 2 hydroxyl | + |
| N‐acetyl‐d‐glucosamine | 221 | 6.3 | 1 amide, 4 hydroxyl, 1 methyl | − |
Open in a separate window
Go to:
5. Conclusions and Perspectives
Interactions between tannins and proteins have been studied for more than 50 years, because of their unique characteristics and potential use in food industry and pharmacology. However, with the new insights regarding regulation of enzymes by tannin concentration and the potential interaction with other non‐protein N compounds, future studies are needed. Special attention should be paid to the use of well‐purified and characterized tannins, because the chemistry of polyphenols and the presence of other compounds in plant extracts may significantly affect tannin interactions with N compounds. Follow‐up studies should aim to extrapolate these results to more complex, heterogenic, realistic systems. In conclusion, studies investigating the interactions between tannins and proteins, but also other organic compounds, are likely to attract significant attention due to the general interest in polyphenols with regard to human health and disease treatment, but also their role in the beverage and food industry.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Bartosz Adamczyk was born in Lodz (Poland) in 1979. He received a Master’s degree in 2003 (Master of Biology) and PhD degree in 2009 (Doctor of Biology), both from the University of Lodz (Poland). After defending his PhD, he started as a Post‐doc at The Finnish Forest Research Institute (Finland). In 2013, he obtained the title of docent (habilitation) from the University of Helsinki (Finland) and started to work there in 2015. His main interests span chemistry of plant secondary compounds, their role in boreal forest ecosystem, plant biochemistry and mitigation of climate change.

Biographical Information
Judy Simon leads the Plant Interactions Ecophysiology Group at the University of Konstanz (Germany). After her studies in biology (RWTH Aachen, Germany), biogeography, soil science and geology (Saarland University, Germany), she conducted her PhD research at the University of Melbourne (Australia). She then worked as a Postdoctoral Fellow at the University of Freiburg (Germany), earning her Habilitation (postdoctoral qualification) in 2013. Since 2014, she conducts her research at the University of Konstanz on the influence of global change on plant interactions with regard to resource allocation strategies (i.e. different N acquisition strategies, N allocation to growth vs. defense) in woody species in boreal, temperate and tropical forest ecosystems.