Healthy Solutions For Your Livestock Production
Insighter® is committed to customers’ customers’in animal GUT Health by providing non-antibiotic antidiarrheal solutions whose results can be seen within 3-7 days.
With our second-generation NGPs (Alternatives to AGPs), we are able to help feed mills and farms get rid of their headaches of diarrheal incidence legitimately, efficaciously and relatively cost-effectively.
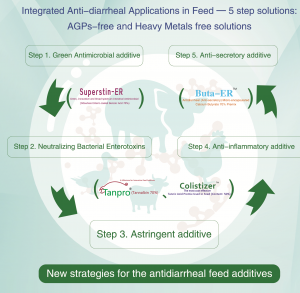
The GUT Health Solutions include:
- Intestinal astringents to replace ZnO : Tannic Acid (Tanpro® & Colistizer®)
- Green & Broad-spectrum Intestinal Antimicrobial to replace Colistin Sulphate: Superstin (Benzoic Acid)
- Anti-secretory antidiarrheal agent : Butye-ER (Calcium Butyrate)
Any doubt? Please feel free to contact us to find out! Seeing is believing!
Deep Insight + Strong Research + Diverse Feed Component Competency x (Optimizing Nutrient Utilization + Supporting the Gut Microbiome) = Superior Solutions